Hepatitis C is the most commonly notified disease in Australia. In 1998 the Hepatitis C Virus Projections Working Group (HCPWG) estimated that there were approximately 210,000 people who had been infected by hepatitis C virus (HCV) in Australia by 2001. Population-based serosurveys are required to validate this estimate. Here we estimate HCV prevalence on the basis of HCV antibody seroprevalence in the Australian national serosurvey. Between 1996 and 1998, 2,800 sera opportunistically collected from pathology laboratories throughout Australia were tested for HCV antibody. National HCV notifications reported from 1991 through 1998 were also assessed. Eighty-one sera were HCV antibody positive, giving an age standardised prevalence of 2.3 per cent (95% CI 1.8%–2.9%). The 20–24 year age group had the highest HCV prevalence, 5.3 per cent (95%CI 3.3%–8.1%) and the male to female ratio was 1.8:1.0. Approximately 111,000 HCV notifications were received from 1991 through 1998. HCV prevalence estimated by the serosurvey is approximately three times higher than cumulative HCV notifications. Age and sex distributions of seroprevalence are broadly consistent with cumulative notification data. These distributions are consistent with the majority of HCV infections in Australia being transmitted by injecting drug use. Very low age specific seroprevalence estimates in the over 50 years age group indicate that there is not a large pool of undiagnosed infection in this age group. The serosurvey provides an estimate of Australian HCV prevalence and baseline data to determine incidence trends, both of which are required for health-care planning. Commun Dis Intell 2004;28:517–521.
Top of page
Introduction
Over the last decade hepatitis C (HCV) has been the most commonly notified infectious disease in Australia.1 The large population of people with HCV, together with estimates of continued high HCV incidence and the often long latency of HCV related disease, will produce an escalating health burden for at least the next two decades.2 The already increasing incidence of hepatocellular carcinoma is thought to be related to the expanding HCV epidemic and cirrhosis due to chronic HCV infection is already the most common underlying reason for liver transplantaion.3,4 The true extent of this epidemic is however not known.
To date estimates of HCV prevalence and incidence have primarily relied on data from specific populations such as prison entrants, injecting drug users, blood product recipients, antenatal populations and blood donors.5–13In 1998 the Hepatitis C Virus Projections Working Group (HCPWG) was formed with a brief to determine estimates of HCV incidence and prevalence in Australia. The HCPWG estimated that there were approximately 210,000 people who had been infected by HCV in Australian by 2001.2 This estimate was primarily derived from estimates of the prevalence of injecting drug use (IDU) and the incidence of HCV infection among IDUs. Population-based serosurveys are required to validate this estimate.
The first Australian national serosurvey of selected infectious diseases was conducted using sera collected in 1996–1998. Here we report the results for HCV antibody testing of specimens from the national serosurvey. We also compare the HCV seroprevalence estimates with the cumulative number of HCV notifications, to assess the potential extent of undiagnosed and unreported HCV infection in Australia.
Top of page
Methods
Serosurvey
Details of the serosurvey are presented elsewhere.14 The samples were collected between July 1996 and December 1998. All major public and private diagnostic laboratories, including reference laboratories, throughout Australia were invited to contribute sera that had been submitted for diagnostic testing and would otherwise have been discarded. Forty-five of the 52 invited laboratories agreed to participate. Sera submitted to laboratories from sexual health clinics were excluded from this study.
All sera were tested by an indirect enzyme immunoassay (EIA), MONOLISA anti-HCV PLUS Version 2 (BIO-RAD, Marnes la Coquette, France) according to the manufacturer’s directions. Samples with an optical density (OD) below that of the cut-off less 10 per cent were deemed negative. All other samples were retested. On retesting, samples giving an OD less than the cut-off were reported as negative. Samples with an OD between that of the cut-off and the cut-off plus 10 per cent recorded as weak positive and all others as positive. The reactivity of samples in the latter two categories was confirmed by a strip immunoblot (using two encoded antigens and three encoded synthetic peptides), CHIRON* RIBA* HCV 3.0 SIA (Chiron Corporation, Emeryville, California, United States of America. Distributed by Ortho-Clinical Diagnostics, Incorporated, Raritan, New Jersey, United States of America) according to the manufacturer’s guidelines. The band patterns were interpreted as negative, indeterminate or positive for HCV infection. Indeterminate sera were subsequently assigned as negative for HCV infection.
Sample sizes were calculated for the following age groups: 1–4, 5–9, 10–14, 15–19, 20–24, 25–29, 30–39, 40–49, 50–59, 60–69 and 70+ years, based on the expected prevalence of hepatitis C antibodies. Within each age group, states and territories were sampled proportionally to their population size. Sample sizes were calculated to achieve confidence intervals of approximately +/-5 per cent for each age group. Approximately equal numbers of sera from males and females were tested. Australian Bureau of Statistics (ABS) end of 1997 population estimates were used to age standardise seroprevalence and to estimate the number of people exposed to HCV.15
Notifications
Hepatitis C notification data (acute and unspecified) was extracted from the National Notifiable Diseases Surveillance System in August 2003 for cases with report date between 1 January 1991 and 31 December 1998 (Personal communication - Communicable Diseases Network Australia - National Notifiable Diseases Surveillance System, 2003). For comparison with the results of the serosurvey, the age distribution of cumulative notifications up to 1998 was determined by calculating age on the 1st of July 1998 based on date of birth or, if not available, the age at notification for each notification. Cumulative notification rates were calculated using the ABS end of 1997 population estimates.15
Confidence intervals for HCV seroprevalence and cumulative notification rates by age group were calculated using exact binomial methods.
Ethics approval
The study was approved by appropriate institutional ethics committees and the State-wide Health Confidentiality and Ethics Committee of the New South Wales Health Department.
Top of page
Results
Of the 2,800 sera tested, 81 were HCV antibody positive, giving an age standardised seroprevalence of 2.3 per cent (95% CI 1.8%–2.9%). This corresponds to approximately 433,000 people (95% CI 336,000 to 530,000) with HCV antibodies, in Australia, in 1998. Approximately 111,000 HCV notifications were reported from 1991 through 1998.
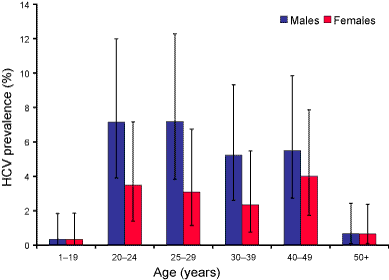
The age distribution for HCV seroprevalence and cumulative notification rate were similar, with the highest rates in the 20 to 49 year age groups and lowest rates in the age groups under 15 years and 50 years and older (Figure 1). However, the highest seroprevalence was in the 20 to 24 year age group, 5.3 per cent (95% CI 3.3%–8.1%) while the highest cumulative notification rate was in the 30 to 39 years age group, 1.1 per cent. There were more seropositive males than females overall (male:female ratio 1.8:1; 95% CI 1.3–3.0). This sex difference was greatest in the 25 to 29 years age group (Figure 2). The cumulative notification rate was also higher in males than in females (male:female ratio 1.7:1).
Figure 1. Age distribution of hepatitis C virus prevalence (percentage of population HCV positive) as determined by serosurvey and cumulative (1991 to 1998) notification rate
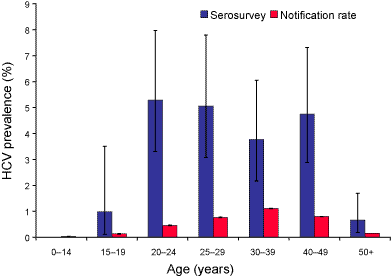
Figure 2. Age distribution of hepatitis C virus prevalence by sex in the HCV serosurvey
Top of page
Discussion
The prevalence estimate from the serosurvey of approximately 433,000 persons with HCV antibodies in Australia in 1998 is almost four fold greater than the 110,000 cumulative notifications reported from 1991 to the end of 1998 and more than double the 196,000 of people estimated to be seropositive in 1997 by mathematical modelling.16
The cumulative HCV notification rate, and to a lesser extent the modelled estimates, are likely to underestimate HCV prevalence. Diagnostic testing for HCV only became available in late 1989 and while notification of HCV commenced in 1991, all states and territories did not report until 1995.1,17 Also, cumulative notifications from 1991 through 1998, underestimate HCV prevalence because of under reporting in the early years of HCV surveillance, low levels of testing in some at risk populations, the generally asymptomatic nature of acute HCV infection, and the long latency of HCV related disease. The modelled estimates of HCV incidence were primarily based on the prevalence of IDU and HCV incidence amongst IDUs.16 Underestimates of either measure would result in an under estimate of population HCV prevalence. An under estimate of the proportion of HCV acquired through non-IDU modes of transmission (around 20% in the HCPWG estimates) two would also produce an underestimate of population HCV prevalence. It is also possible that the serosurvey overestimates HCV prevalence.
Anonymous opportunistic testing of remanent sera has been shown to provide estimates of immunity comparable with a random population based survey of some vaccine preventable diseases.18,19 However, the lack of detailed information about participants in opportunistic serosurveys means that it is not possible to identify or control for various potential biases. In this serosurvey, the potential for selection bias was reduced by enrolling 87 per cent of major laboratories and including primarily ambulatory rather than hospitalised patients.14 The majority of Australian laboratories are located in major centres which may have resulted in selection bias. However, a comparison of Accessibility and Remoteness Index Australia scores for the population tested in the serosurvey and the Australian population showed that remote areas were not under-represented (data not shown). It is possible that people with chronic HCV were over sampled due to their potentially higher utilisation of health care services. The effect of this bias would be partially ameliorated by exclusion of sera from sexual health clinics but ideally sera from liver clinics should also have been excluded. Further, while this bias would result in an over estimation of overall prevalence, it should not distort the pattern of HCV seroprevalence by age or sex for HCV.
The low seroprevalence in persons over the age of 50 years in this study is consistent with findings from an opportunistic serosurvey in England and Wales and a population based random survey in the United States of America.20,21 In both of these surveys, seroprevalence was found to be associated with drug use. In contrast, a study from Italy in which seroprevalence was highest in older age groups, showed an association with medical interventions.22 The low levels of notification and seroprevalence in older age groups in Australia indicate that these age groups are not particularly subject to under notification and there is unlikely to be a hidden epidemic related to medical interventions in older Australians. A more robust estimate of HCV seroprevalence and conclusive evidence that IDU is the main source of infection in Australia, could be ascertained by undertaking a population based random sampled serosurvey in which risk factor information is collected. However, such surveys are costly and can also be biased by non-participation especially by high-risk groups such as IDUs.
The impact on the health system of illness associated with chronic HCV infection over the next decade is likely to increase as those aged 20 to 50 in this study approach the duration of infection, 15–25 years, at which severe complications of hepatitis C arise.2,23 The projected high prevalence of such complications, makes the planning of health services to provide appropriate and accessible services and treatment, including antiviral therapy, imperative.
Top of page
Acknowledgements
NCIRS is supported by the Australian Government Department of Health and Ageing, The NSW Department of Health and The Children’s Hospital at Westmead. NCHECR is supported by the Australian Government Department of Health and Ageing. We thank the staff of the 45 laboratories who provided the sera (http://immunise.health.gov.au/metadata/measeval.htm pages 8–9), laboratory staff at the ICPMR and the nurses at the RAHC Centre for Immunisation Research, Westmead, for their help in processing and testing the sera.
Top of page
References
1. Blumer C, Roche P, Spencer J, Lin M, Milton A, Bunn C, et al. Australia's notifiable diseases status, 2001: annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell 2003;27:1–78.
2. Law MG, Dore GJ, Bath N, Thompson S, Crofts N, Dolan K, et al. Modelling hepatitis C virus incidence, prevalence and long-term sequelae in Australia, 2001. Int J Epidemiol 2003;32:717–724.
3. Brotodihardjo AE, Tait N, Weltman MD, Liddle C, Little JM, Farrell GC. Hepatocellular carcinoma in western Sydney. Aetiology, changes in incidence, and opportunities for better outcomes. Med J Aust 1994;161:433–435.
4. NCHECR NCiHEaCR. HIV/AIDS, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2003. Sydney: National Centre in HIV Epidemiology and Clinical Research, The University of New South Wales, 2003.
5. MacDonald MA, Wodak AD, Dolan KA, van Beek I, Cunningham PH, Kaldor JM. Hepatitis C virus antibody prevalence among injecting drug users at selected needle and syringe programs in Australia, 1995–1997. Collaboration of Australian NSPs. Med J Aust 2000;172:57–61.
6. Butler T, Spencer J, Cui J, Vickery K, Zou J, Kaldor J. Seroprevalence of markers for hepatitis B, C and G in male and female prisoners—NSW, 1996. Aust N Z J Public Health 1999;23:377–384.
7. Ogilvie EL, Veit F, Crofts N, Thompson SC. Hepatitis infection among adolescents resident in Melbourne Juvenile Justice Centre: risk factors and challenges. J Adolesc Health 1999;25:46–51.
8. Butler TG, Dolan KA, Ferson MJ, McGuinness LM, Brown PR, Robertson PW. Hepatitis B and C in New South Wales prisons: prevalence and risk factors. Med J Aust 1997;166:127–130.
9. Archer GT, Buring ML, Clark B, Ismay SL, Kenrick KG, Purusothaman K, et al. Prevalence of hepatitis C virus antibodies in Sydney blood donors. Med J Aust 1992;157:225–227.
10. Leslie DE, Rann S, Nicholson S, Fairley CK, Gust ID. Prevalence of hepatitis C antibodies in patients with clotting disorders in Victoria. Relationship with other blood borne viruses and liver disease. Med J Aust 1992;156:789–792.
11. Crofts N, Aitken CK. Incidence of bloodborne virus infection and risk behaviours in a cohort of injecting drug users in Victoria, 1990–1995. Med J Aust 1997;167:17–20.
12. Crofts N, Stewart T, Hearne P, Ping XY, Breshkin AM, Locarnini SA. Spread of bloodborne viruses among Australian prison entrants. BMJ 1995;310:285–288.
13. Williamson G, Wilson J, Low J. Prevalence of infection with hepatitis C in Geelong. Med J Aust 1990;152:504.
14. Gidding H. Australia's national serosurveillance program. NSW Public Health Bull 2003;14:90–93.
15. Australian Bureau of Statistics. Australian Demographic Statistics, December Quarter 1997. Australian Bureau of Statisitics, Canberra, Australia, 1998.
16. Law MG. Modelling the hepatitis C virus epidemic in Australia. Hepatitis C Virus Projections Working Group. J Gastroenterol Hepatol 1999;14:1100–1107.
17. Kuo G, Choo QL, Alter HJ, Gitnick GL, Redeker AG, Purcell RH, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 1989;244:362–364.
18. Osborne K, Gay N, Hesketh L, Morgan-Capner P, Miller E. Ten years of serological surveillance in England and Wales: methods, results, implications and action. Int J Epidemiol 2000;29:362–368.
19. Kelly H, Riddell MA, Gidding HF, Nolan T, Gilbert GL. A random cluster survey and a convenience sample give comparable estimates of immunity to vaccine preventable diseases in children of school age in Victoria, Australia. Vaccine 2002;20:3130–3136.
20. Balogun MA, Ramsay ME, Hesketh LM, Andrews N, Osbourne KP, Gay NJ, et al. The prevalence of hepatitis C in England and Wales. J Infect 2002;45:219–226.
21. Alter MJ, Kruszon-Moran D, Nainan OV, McQuillan GM, Gao F, Moyer LA, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556–562.
22. Guadagnino V, Stroffolini T, Rapicetta M, Costantino A, Kondili LA, Menniti-Ippolito F, et al. Prevalence, risk factors, and genotype distribution of hepatitis C virus infection in the general population: a community-based survey in southern Italy. Hepatology 1997;26:1006–1011.
23. Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology 2001;34:809–816.
Top of page
Author affiliations
1. National Centre in HIV Epidemiology and Clinical Research, University of New South Wales, Sydney, New South Wales
2. National Centre for Immunisation Research and Surveillance of Vaccine Preventable Diseases and the University of Sydney, Sydney, New South Wales
3. Centre for Infectious Diseases and Microbiology, Institute of Clinical Pathology and Medical Research, Westmead, New South Wales
Corresponding author: Ms Janaki Amin, National Centre in HIV Epidemiology and Clinical Research, Level 2, 376 Victoria St, Sydney NSW 2010 Australia. Telephone: +61 2 9385 0900. Facsimile: +61 2 9385 0920. Email: jamin@nchecr.unsw.edu.au
This article was published in Communicable Diseases Intelligence Vol 28 No 4, December 2004.
