In 2005 there were 345 laboratory-confirmed cases of invasive meningococcal disease (IMD) analysed by the National Neisseria Network, a nationwide network of reference laboratories. The phenotypes (serogroup, serotype and serosubtype) and antibiotic susceptibility of 214 isolates of Neisseria meningitidis from invasive cases of meningococcal disease were determined and an additional 131 cases were confirmed by non-culture-based methods. Nationally, 251 (73%) confirmed cases were infected with serogroup B and 50 (14.5%) with serogroup C meningococci. The total number of confirmed cases was 16 fewer than the 361 cases identified in 2004. The number of cases decreased in Queensland, Tasmania, New South Wales and the Australian Capital Territory and increased slightly in Victoria, South Australia, Western Australia and the Northern Territory. The age distribution of IMD showed a typical primary peak in those aged four years or less with a lower secondary peak in adolescents and young adults. Serogroup B cases were 90 per cent of all cases in those aged four years or less and 75 per cent in those aged 15–24 years. The proportion of all invasive disease represented by serogroup C disease was highest in the 20–24 years and older age groups. The common phenotypes circulating in Australia were B:15:P1.7 and C:2a:P1.5. However significant jurisdictional differences in the serogroup and phenotypic distribution of meningococci were again evident and considerable heterogeneity of subtypes was noted. No evidence of meningococcal capsular ‘switching’ or genetic recombination was detected. About two thirds of all isolates showed decreased susceptibility to the penicillin group of antibiotics (MIC 0.06–0.5 mg/L). A single isolate was penicillin resistant at 1 mg/L and another was rifampicin resistant. Commun Dis Intell 2006;30:211–221.
Top of page
Introduction
Laboratory confirmation of the clinical diagnosis of invasive meningococcal disease (IMD) is desirable, not only for the certainty provided for the management of the individual patient, but also to assist in the application of public health measures for disease control. The National Neisseria Network (NNN) is a collaborative national program of reference laboratories in each state and territory of Australia. NNN examines the recognition, antimicrobial resistance surveillance and typing of invasive meningococci, including both isolate-based and non-culture derived methodologies. The NNN began in 1994 and relied solely on data derived from examination of isolates from culture-positive cases of IMD. Subsequently this information has been complemented by non-culture based-methods.
A publicly funded program of vaccination of children and adolescents with serogroup C conjugate vaccine was commenced in 2003 and was fully operational in 2004. This report analyses information gathered by the NNN on laboratory-confirmed cases of IMD in the calendar year 2005. It follows the format used for the 2004 annual report published in Communicable Diseases Intelligence1 in that data on all laboratory-confirmed cases was aggregated for analysis. Prior to 2004 data on IMD diagnosed by culture-based and non-culture methods were provided separately.2–11
Top of page
Methods
The NNN is a long-term collaborative program for the laboratory surveillance of the pathogenic Neisseria, N. meningitidis and N. gonorrhoeae.1–11 A network of reference laboratories in each state and territory (laboratories are listed in the acknowledgements) performs and gathers laboratory data on cases of IMD throughout Australia.
Isolate-based invasive meningococcal disease cases
Each case confirmation was based upon isolation of a meningococcus from a normally sterile site and defined as IMD according to Public Health Laboratory Network definitions. Information on the site of infection, the age and sex of the patient and the outcome (survived/died) of the infection was sought. The isolate-based subset of the program categorised cases on the basis of site of isolation of the organism. Where an isolate was grown from both blood and cerebrospinal fluid (CSF) cultures in the same patient, the case was classified as one of meningitis. It is recognised that total number of cases and particularly the number of cases of meningitis e.g. where there was no lumbar puncture or else where lumbar puncture was delayed and the culture sterile, is underestimated. However the above approach has been used since the beginning of this program and is continued for comparative purposes.
Phenotyping of invasive isolates of meningococci by serotyping and serosubtyping was based on the detection of outer membrane protein (porin) antigens using a standard set of monoclonal antibodies obtained from the National Institute for Public Health, The Netherlands. Increasingly, sequencing of products derived from amplification of the porin genes porA and porB has been used to supplement and supplant serotyping analyses based on the use of monoclonal antibodies. For the purposes of continuity and comparability, the typing data from both approaches has been unified in the accompanying tables by converting sequence data to the more familiar serotyping/serosubtyping nomenclature.
Antibiotic susceptibility was assessed by determining the minimal inhibitory concentration (MIC) to antibiotics used for therapeutic and prophylactic purposes. This program uses the following parameters to define the various levels of penicillin susceptibility or resistance when determined by a standardised agar plate dilution technique.12
sensitive, MIC ≤ 0.03 mg/L.
less sensitive, MIC 0.06–0.5 mg/L.
relatively resistant MIC ≤ 1 mg/L.
Strains with MICs which place them in the category of ‘sensitive’ or ‘less sensitive’ would be considered to be amenable to penicillin therapy when used in currently recommended doses. However precise MIC/outcome correlations are difficult to obtain because of the nature of IMD.
Non-culture-based laboratory-confirmed cases
Additional laboratory confirmation of suspected cases of IMD was obtained by means of non-culture based methods including nucleic acid amplification (NAA) and serological techniques. NAA testing is essentially by polymerase chain reaction (PCR) techniques13 and has been progressively introduced in the different jurisdictions. Data from the results of these investigations were included for the first time in the 1999 report. The serological results are based on results of tests performed using the methods and test criteria of the Manchester PHLS reference laboratory, United Kingdom as assessed for Australian conditions.14–16 Where age, sex and outcome data for patients with non-culture-based diagnoses are available these were also recorded. The site of a sample of a positive NAA is also used to define the clinical syndrome. This separation is not possible for cases diagnosed serologically.
Top of page
Results
Aggregated data on cases confirmed by culture-based and non-culture-based methods
Number of laboratory-confirmed cases
There were 345 instances of laboratory-confirmed cases of IMD in 2005 (Table 1) compared with 361 in 2004 and 494 in 2003. In 214 cases a positive culture was obtained with or without a positive non-culture-based test and 131 cases were confirmed by a non-culture-based method alone. The total number of all laboratory-confirmed cases changed slightly in all jurisdictions in 2005 when compared to 2004 data. The largest decrease in numbers was in Queensland (to 58 from 75) with smaller numerical decreases in New South Wales (11 fewer cases), the Australian Capital Territory (2 less) and Tasmania (7 less). There were nine more cases in South Australia, seven in Western Australia, four in Victoria and one more in the Northern Territory.
Table 1. Number of laboratory confirmed cases of invasive meningococcal disease, Australia, 2005, by state or territory and serogroup
State or territory |
Serogroup |
Total |
| |
B |
C |
A |
Y |
W135 |
NG* |
|
| ACT |
4 |
3 |
|
|
2 |
|
9 |
| NSW |
69 |
19 |
|
3 |
9 |
12 |
112 |
| NT |
5 |
3 |
|
|
|
|
8 |
| Qld |
44 |
13 |
|
1 |
|
|
58 |
| SA |
18 |
3 |
|
1 |
|
1 |
23 |
| Tas |
9 |
1 |
|
|
|
|
10 |
| Vic |
61 |
7 |
1 |
3 |
3 |
5 |
80 |
| WA |
41 |
1 |
|
2 |
|
1 |
45 |
| Australia |
251 |
50 |
1 |
10 |
14 |
19 |
345 |
Seasonality
Sixty-one (17.6%) cases occurred between 1 January and 31 March, 63 (18.2%) between 1 April and 30 June, 126 (36.5%) between 1 July and 30 September and 95 (27.5%) between 1 October and 31 December. A winter peak of meningococcal disease is usual.
Top of page
Age distribution
Nationally, the peak incidence of meningococcal disease was again in those aged 4 years or less (Table 2, Figure 1). Those aged less than one year or in the 1–4 age group accounted for 47 (13.6%) and 63 (18.2%) cases respectively. These numbers are virtually identical to the total number and proportions recorded in these age groups in 2004. The combined total of cases confirmed by all methods in these two groups (110) was one less than that recorded in 2004 and this age grouping accounted for 31.8 per cent of all laboratory-confirmed cases, again a proportion little different from the 30.9 per cent reported in 2004. A secondary disease peak is also usual in the 15–19 years age group. The total of 48 cases (14% of all confirmed cases) in this age group in 2005 was less than the 61 (17%) cases seen in 2004 and the 89 (18%) seen in 2003. Those aged 15–24 years, together accounted for 88 (23.4%) cases (96 cases, 26.7%, in 2004).
Figure 1. Number of serogroup B and C cases of invasive meningococcal disease confirmed by all methods, Australia, 2005, by age group
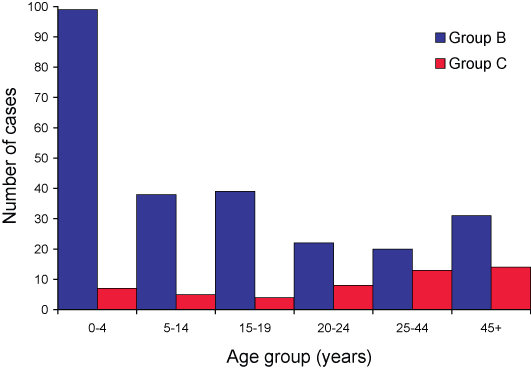
Table 2. All laboratory confirmed cases of invasive meningococcal disease, Australia, 2005, by age, state or territory and serogroup
State or territory |
Serogroup |
Age group |
Total |
| |
|
<1 |
1–4 |
5–9 |
10–14 |
15–19 |
20–24 |
25–44 |
45–64 |
65+ |
NS |
|
| ACT |
B |
0 |
1 |
0 |
0 |
1 |
0 |
2 |
0 |
0 |
1 |
4 |
| |
C |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
0 |
0 |
0 |
3 |
| |
Total |
0 |
2 |
0 |
0 |
2 |
2 |
2 |
1 |
0 |
0 |
9 |
| NSW |
B |
12 |
13 |
4 |
5 |
11 |
8 |
7 |
3 |
5 |
1 |
69 |
| |
C |
2 |
1 |
1 |
1 |
1 |
3 |
9 |
1 |
0 |
0 |
19 |
| |
Total |
15 |
17 |
6 |
8 |
14 |
13 |
19 |
7 |
12 |
1 |
112 |
| NT |
B |
2 |
1 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
5 |
| |
C |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
3 |
| |
Total |
2 |
1 |
1 |
0 |
0 |
0 |
3 |
1 |
0 |
0 |
8 |
| Qld |
B |
8 |
9 |
5 |
6 |
5 |
3 |
3 |
4 |
0 |
1 |
44 |
| |
C |
0 |
2 |
0 |
0 |
2 |
2 |
0 |
7 |
0 |
0 |
13 |
| |
Total |
8 |
11 |
5 |
6 |
7 |
5 |
3 |
11 |
1 |
1 |
58 |
| SA |
B |
3 |
4 |
4 |
0 |
2 |
2 |
1 |
0 |
2 |
0 |
18 |
| |
C |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
3 |
| |
Total |
3 |
4 |
6 |
0 |
2 |
2 |
2 |
1 |
3 |
0 |
23 |
| Tas |
B |
2 |
3 |
3 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
9 |
| |
C |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| |
Total |
2 |
3 |
4 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
10 |
| Vic |
B |
8 |
9 |
3 |
6 |
14 |
7 |
3 |
10 |
1 |
0 |
61 |
| |
C |
0 |
1 |
0 |
0 |
0 |
1 |
2 |
2 |
1 |
0 |
7 |
| |
Total |
8 |
10 |
6 |
6 |
14 |
10 |
8 |
14 |
4 |
0 |
80 |
| WA |
B |
9 |
15 |
2 |
0 |
5 |
2 |
2 |
6 |
0 |
0 |
41 |
| |
C |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
| |
Total |
9 |
15 |
3 |
0 |
7 |
2 |
2 |
7 |
0 |
0 |
45 |
| Australia |
B |
44 |
55 |
21 |
17 |
39 |
22 |
20 |
23 |
8 |
2 |
251 |
| |
C |
2 |
4 |
4 |
1 |
4 |
8 |
13 |
13 |
1 |
0 |
50 |
| |
other |
1 |
4 |
6 |
2 |
5 |
3 |
7 |
5 |
11 |
0 |
44 |
| Total |
|
47 |
63 |
31 |
20 |
48 |
33 |
40 |
41 |
20 |
2 |
345 |
| % of all |
|
13.6 |
18.2 |
9 |
5.8 |
13.9 |
9.5 |
11.5 |
11.9 |
5.8 |
0.8 |
|
Top of page
Serogroup data
The serogroup of the meningococci causing disease was determined in 326 of the 345 laboratory confirmed cases of IMD in 2005. Two hundred and fifty-one (76.9%) were of serogroup B, 50 (15.3%) of serogroup C, 1 of serogroup A, 10 (3%) of serogroup Y and 14 (4.3%) of serogroup W135. The serogroup was not determined in two of the 214 cases confirmed by culture, in 6 of 111 cases confirmed by NAA or in 11 of the 20 serologically-confirmed cases. In 2004, a total of 243 (73%) cases of serogroup B and 71 (21%) of serogroup C IMD were confirmed from a total of 361 laboratory-confirmed cases. The corresponding data for 2003 were a total of 285 (58%) cases of serogroup B and 155 (31%) of serogroup C IMD identified from a total of 494 laboratory-confirmed cases.
The serogroup distribution varied with age (Figure 1) and jurisdiction (Table 2), as in previous years. Traditionally, serogroup B disease is concentrated in younger age groups with serogroup C infections increasing as a proportion of all isolates in adolescents and young adults (Figure 2).
Figure 2. Serogroup B and C meningococcal disease as a percentage of cases of invasive meningococcal disease confirmed by all methods, Australia, 2005, by age group
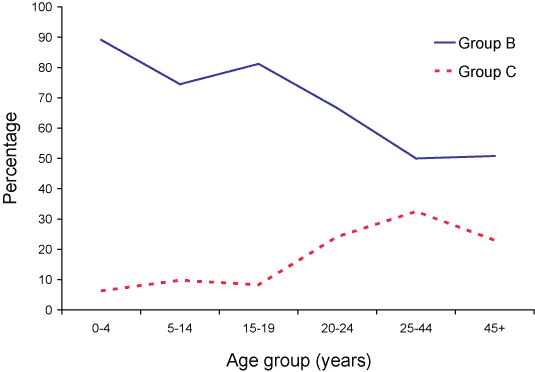
In 2005, serogroup B meningococci predominated in all age groups in aggregated national data. Ninety-nine (90%) of the total of 110 laboratory-confirmed IMD cases in those aged less than four years were serogroup B and 6 (5.5%) were serogroup C. These figures differ little from 2004 data. In 2004, 97 (88%) of the total of 111 laboratory-confirmed IMD cases in those aged less than 4 years were serogroup B and 6 (5.5%) were serogroup C.
In those aged 5 to 14 years, 38 serogroup B meningococcal cultures represented 74 per cent of the 51 confirmed cases and the 5 cases of serogroup C represented 10 per cent. The number of serogroup B cases in this age group increased from the 27 identified in 2004, but the serogroup C case numbers were unaltered.
There were 48 cases of IMD confirmed nationally in those aged 15–19 years in 2005 (61 in 2004). These comprised 39 (81%) serogroup B and 5 (10%) serogroup C cases. In 2004 there were 40 (67%) serogroup B cases and 17 (28%) serogroup C infections in this age group.
There were 33 instances of IMD in those aged 20–24 years in 2005, 22 (67%) with serogroup B and 8 (24%) with serogroup C meningococci. In 2004, the number of infections and their distribution was not dissimilar with 35 infections; 20 (57%) serogroup B and 11 (35%) serogroup C.
In older age groups (25 years and above), there were 101 laboratory-confirmed cases of IMD in 2005, of which half were serogroup B and a quarter serogroup C. Again these data were closely similar to data from 2004.
A comparison of data from 2005 and 2004 shows little change in serogroup B numbers, except for an increase from 27 to 38 cases in those aged 5–14 years and a smaller decrease in those aged 25 years or more (Table 3). Similarly, there was little further change in the number of serogroup C cases except in those aged 15–19 years. There were only four laboratory-confirmed cases of serogroup C IMD in this age group in 2005 compared with 17 in 2004 and 36 in 2003.
Table 3. A comparison of the number and proportion of serogroup B and serogroup C laboratory-confirmed cases of invasive meningococcal disease, 2004 and 2005, by age
Year |
Serogroup |
Age group (years) |
| |
|
<4 |
5–14 |
15–19 |
20–24 |
25+ |
| 2005 |
B |
99 (90%) |
38 (75%) |
39 (81%) |
22 (67%) |
51 (50%) |
| |
C |
6 (5.5%) |
5 (10%) |
4 (8%) |
8 (24%) |
27 (27%) |
| |
All |
110 |
51 |
48 |
33 |
101 |
| 2004 |
B |
97 (88%) |
27 (77%) |
40 (65%) |
20 (57%) |
59 (50%) |
| |
C |
6 (5.5%) |
5 (14%) |
17 (28%) |
11 (31%) |
32 (27%) |
| |
All |
110 |
35 |
61 |
35 |
117 |
Jurisdictional differences in the distribution of serogroup B and C meningococcal cases continued in 2005 (Table 1). Serogroup B infections predominated nationally and in all jurisdictions. In New South Wales there was little change between 2004 and 2005 in the number and proportion of all serogroup B and C cases. In Queensland, while the total number of IMD cases decreased, the proportion of serogroup B and C infections was unchanged. In Victoria, the total number of cases was also little changed from 2004, but the number and proportion of serogroup B cases increased and that of serogroup C decreased further. In both the Australian Capital Territory and Tasmania there had been clusters of serogroup C cases in earlier years. The number of all cases remained low and in 2005 serogroup C cases declined in number. South Australia, Western Australia and the Northern Territory have had a marked preponderance of serogroup B cases for many years. There were three serogroup C cases of a total of 23 infections in South Australia in 2005 (a single case serogroup C case in 2004), a single case serogroup C infection of a total of 45 cases in Western Australia (6 in 2004) and three of eight cases were serogroup C in the Northern Territory (1 in 2004).
Top of page
Outcome data for invasive meningococcal disease for all laboratory-confirmed cases of invasive meningococcal disease
Outcome data (survived or died) were available for 163 (47%) of the 346 laboratory-confirmed cases (Table 4). Fifteen deaths were recorded in this group (9.2%). Outcomes were available for 117 of 251 (47%) serogroup B infections and 20 of 51 (39%) serogroup C infections. There were 10 (8.5%) deaths in serogroup B infections and 3 (15%) in serogroup C infections.
Table 4. Outcome data (survived, died) for laboratory-confirmed cases of invasive meningococcal disease, Australia, 2005, by syndrome and serogroup
Disease type |
Outcome |
Serogroup |
Total |
| B |
C |
Y |
W135 |
NG |
| Meningitis |
Survived |
41 |
0 |
2 |
0 |
0 |
43 |
| |
Died |
2 |
0 |
0 |
0 |
0 |
2 |
| |
Total |
43 |
0 |
2 |
0 |
0 |
45 |
| Septicaemia |
Survived |
66 |
17 |
4 |
5 |
13 |
105 |
| |
Died |
8 |
3 |
1 |
1 |
0 |
13 |
| |
Total |
74 |
20 |
5 |
6 |
13 |
118 |
| All cases |
Survived |
107 |
17 |
6 |
5 |
13 |
148 |
| |
Died |
10 |
3 |
1 |
1 |
0 |
15 |
| |
Total |
117 |
20 |
7 |
6 |
13 |
163 |
There were two deaths in 45 patients (4.4%) with meningitis; both of these patients were infected with a serogroup B strain. Thirteen deaths were recorded in 118 bacteraemic patients (11%). There were 74 cases of serogroup B meningococcal bacteraemia with 8 (10.8%) deaths and 20 cases were caused by serogroup C strains among whom three fatalities were recorded (15%). A single fatality was recorded in the seven serogroup Y cases and another among the six instances of serogroup W135 bacteraemia.
Phenotypes of invasive meningococcal isolates
Examination of the phenotype of invasive isolates by determination of their serogroup, serotype and serosubtype revealed considerable heterogeneity especially in the serogroup B isolates. The predominant serotypes/serosubtypes in each state and territory are shown in Table 5. Serogroup B meningococci are in general more difficult to characterise by serological methods and a number could not be phenotyped. A total of 15 isolates of the B:4:P1.4 phenotype were identified in the Australian Capital Territory, Victoria, New South Wales, Queensland and South Australia in 2005. The number of isolates of this phenotype, circulating in New Zealand at high rates for many years, have declined in recent years in Australia. Forty-one meningococci of this phenotype were detected in 2002, 25 in 2003 and 19 in 2004. Historically, the other common phenotype circulating has been B:15:P1.7. In 2004, eight strains of this type were seen and were concentrated in New South Wales. In 2005, this was the commonest phenotype detected nationally with a total of 16 examples, detected in the Australian Capital Territory, Victoria, New South Wales, Queensland and Western Australia. There is continuing interest in the presence of any serogroup B meningococci of serotypes 2a or 2b but no serogroup B strains of these serotypes were detected in 2005.
Table 5. Common serotypes and serosubtypes of isolates from culture-positive cases of Neisseria meningitidis infection, Australia, 2005, by state or territory
State or territory |
Serogroup B |
|
Serogroup C |
| Serotype |
n |
Serosubtype |
n |
|
Serotype |
n |
Serosubtype |
n |
| ACT |
4 |
2 |
1.7 |
1 |
|
2a |
3 |
1.4 |
2 |
| |
|
|
1.16 |
1 |
|
|
|
nst |
1 |
| |
15 |
1 |
1.7 |
1 |
|
|
|
|
|
| NSW |
4 |
15 |
1.4 |
7 |
|
2a |
8 |
1.5 |
6 |
| |
|
|
1.14 |
2 |
|
|
|
1.4 |
1 |
| |
|
|
1.15 |
3 |
|
|
|
nst |
1 |
| |
|
|
1.7 |
2 |
|
|
|
|
|
| |
|
|
nst |
1 |
|
nt |
4 |
1.12 |
1 |
| |
15 |
6 |
1.7 |
4 |
|
|
|
1.12,13 |
1 |
| |
|
|
1.7,16 |
1 |
|
|
|
nst |
2 |
| |
|
|
nst |
1 |
|
|
|
|
|
| |
1 |
5 |
1.14 |
3 |
|
|
|
|
|
| |
|
|
nst |
2 |
|
|
|
|
|
| |
nt |
17 |
1.4 |
3 |
|
|
|
|
|
| |
|
|
1.7 |
2 |
|
|
|
|
|
| |
|
|
1.15 |
1 |
|
|
|
|
|
| |
|
|
1.9 |
2 |
|
|
|
|
|
| |
|
|
nst |
9 |
|
|
|
|
|
| NT |
14 |
1 |
nst |
1 |
|
nt |
1 |
1.15 |
1 |
| Qld |
4 |
4 |
1.4 |
3 |
|
2a |
11 |
1.4 |
1 |
| |
|
|
1.7 |
1 |
|
|
|
1.5 |
6 |
| |
15 |
6 |
1.7 |
4 |
|
|
|
1.5,2 |
2 |
| |
|
|
1.6 |
1 |
|
|
|
nst |
2 |
| |
|
|
nst |
1 |
|
nt |
1 |
1.5,2 |
1 |
| |
1 |
4 |
1.14 |
3 |
|
|
|
|
|
| |
|
|
nst |
1 |
|
|
|
|
|
| |
nt |
18 |
1.4 |
5 |
|
|
|
|
|
| |
|
|
1.14 |
3 |
|
|
|
|
|
| |
|
|
1.12,13 |
1 |
|
|
|
|
|
| |
|
|
1.13 |
1 |
|
|
|
|
|
| |
|
|
nst |
7 |
|
|
|
|
|
| SA |
15 |
5 |
1.16 |
4 |
|
2a |
1 |
1.5,2 |
1 |
| |
|
|
nst |
1 |
|
|
|
|
|
| |
14 |
1 |
nst |
1 |
|
|
|
|
|
| |
4 |
1 |
1.4 |
1 |
|
|
|
|
|
| |
nt |
4 |
1.14 |
4 |
|
|
|
|
|
| Tas |
4 |
2 |
1.19,15 |
2 |
|
|
|
|
|
| Vic |
4,7 |
7 |
1.4 |
3 |
|
2a |
5 |
1.4 |
4 |
| |
|
|
others |
3 |
|
|
|
1.5,2 |
1 |
| |
|
|
nst |
1 |
|
2b |
1 |
nst |
1 |
| |
7 |
2 |
1.7,16 |
1 |
|
|
|
|
|
| |
|
|
nst |
1 |
|
|
|
|
|
| |
15 |
8 |
1.7 |
6 |
|
|
|
|
|
| |
|
|
1.7,16 |
2 |
|
|
|
|
|
| |
17,7 |
4 |
nst |
4 |
|
|
|
|
|
| |
19 |
6 |
various |
6 |
|
|
|
|
|
| |
nt |
3 |
1.3 |
2 |
|
|
|
|
|
| |
|
|
nst |
1 |
|
|
|
|
|
| WA |
1 |
1 |
1.14 |
1 |
|
|
|
|
|
| |
14 |
3 |
nst |
3 |
|
|
|
|
|
| |
15 |
1 |
1.7 |
1 |
|
|
|
|
|
| |
nt |
17 |
various |
7 |
|
|
|
|
|
| |
|
|
nst |
10 |
|
|
|
|
|
Among serogroup C strains, phenotype C:2a:P1.4 is of particular interest. This phenotype has figured prominently in Victorian data in recent years. In 2003 there were 29 and in 2004, 21 serogroup C isolates of this serotype/serosubtype. Only eight were seen in 2005 and these were detected in the Australian Capital Territory, Victoria, New South Wales and Queensland, all in low numbers. All except one of the serotypeable serogroup C isolates were 2a. The most frequently detected 2a serosubtype, 1.5, was present only in New South Wales and Victoria.
Top of page
Anatomical source of samples for laboratory-confirmed cases
Table 6 shows the source of clinical samples by which laboratory confirmation of IMD was obtained. Those diagnoses shown as culture positive may have had positive PCR and/or serology, those shown as PCR positive were culture negative with or without positive serology and those shown as serologically positive were culture and PCR negative. There were 51 isolates from CSF either alone or with a blood culture isolate and 160 from blood cultures alone. There were three other isolates from synovial fluid. The ratio of CSF isolates to blood-culture isolates was 0.3:1. For PCR based diagnosis, this ratio was 0.93:1. This probably reflects the capacity of PCR to amplify meningococcal DNA even after antibiotic treatment and/or delayed lumbar puncture.17
Table 6. Anatomical source of samples positive for a laboratory-confirmed case of invasive meningococcal disease, Australia, 2005
Specimen type |
Isolate of meningococci |
PCR positive* |
Total |
| Blood |
160 |
57 |
217 |
| CSF +/– blood |
51 |
53 |
104 |
| Other† |
3 |
1 |
4 |
| Serology alone‡ |
|
|
20 |
| Total |
214 |
111 |
345 |
Antibiotic susceptibility surveillance of invasive meningococcal isolates
Penicillin
Two hundred and six isolates were available for determination of their susceptibility to penicillin. Using defined criteria, a single isolate from CSF was resistant to penicillin at an MIC of 1 mg/L, 140 isolates (68%) were less sensitive to penicillin in the MIC range 0.06–0.5 mg/L and 65 (31.5%) fully sensitive (MIC 0.03 mg/L or less). These proportions are similar to those observed in recent years. Eleven isolates had MICs of 0.5 mg/L; blood cultures (5), CSF (5) and joint fluids (1).
Other antibiotics
All isolates were fully susceptible to ceftriaxone (and by extrapolation to other third generation cephalosporins) and to the prophylactic agents ciprofloxacin and rifampicin, with the exception of a single isolate with an MIC for rifampicin of 1 mg/L.
Top of page
Discussion
There was a further decline, albeit slight, in the number of laboratory-confirmed cases of IMD in Australia in 2005. Numbers declined most in Queensland but also in Tasmania and the Australian Capital Territory where some case clusters had been seen in recent years. Cultures were obtained from sterile sites in 214 cases, the lowest number of isolates available since 1994. Non-culture based diagnoses were used to confirm 131 (38%) of cases IMD.
The distribution of cases of IMD in Australia shows major differences when considered by jurisdiction, age and serogroup of the infecting organism and these were again present in 2005. Western and South Australia have had a preponderance of serogroup B infections for many years, Victoria, Tasmania and the Australian Capital Territory until recently tended to have a greater proportion of serogroup C infections than New South Wales or Queensland. Nationally, serogroup B infections were five times more common than serogroup C IMD. This differential was greater in Western and South Australia as in previous years, and in 2005 also in Victoria and Tasmania. Nearly 40 per cent of all serogroup C disease was in New South Wales and another 25 per cent of the national total occurred in Queensland. The Australian Capital Territory and the Northern Territory had low numbers of cases but serogroup C infections remained prominent.
Serogroup B infections have been more frequently encountered in younger age groups where there is a primary peak in IMD infection rates, and in 2005, 90 per cent of 110 confirmed infections in those aged four years or less were with serogroup B. This proportion is little changed from 2005. Serogroup C infections were again infrequent in this age group (Table 3). NNN reports have consistently noted that in the usual secondary peak in IMD in adolescents and young adults, the proportion of serogroup C infections increased over those present in younger age groups. Table 3 shows that this pattern had changed in 2005 from 2004 and earlier years with both the number and proportion of serogroup C cases in the 15–19 years age group now lower. Although overall numbers of cases are low, the same proportional decrease from 2004 to 2005 was not evident in those aged 20–24 years or in those aged 25 years or more. However in those aged 20–24 years, the number of serogroup C infections had already declined from 35 in 2003 to 11 in 2004. There were 41 serogroup C cases identified in those aged 15–19 years in 2003.
The NNN is not as well placed as others to analyse the effect of the national vaccination program with serogroup C conjugate vaccine for reasons previously discussed.10 These included differences between clinical and laboratory surveillance case definitions, the different rates of introduction and use of non-culture-based confirmatory tests over time and the influence of clinical practice on laboratory-based diagnosis. These concerns remain and fluctuations in the rates of IMD can occur naturally or be influenced by rates of intercurrent viral infection. The data available here and in previous reports will hopefully assist in this formal assessment.
Some concerns have been expressed that the well established ability of Neisseria meningitidis to undergo substantial genetic reconfiguration by a number of mechanisms may pose threats to the longer term efficacy of monovalent capsular vaccines. While there is some evidence that some meningococci isolated and examined by the NNN in recent years show evidence of genetic recombination, these existing strains have not proliferated and no new combinations were encountered in 2005. Analysis of meningococcal subtypes and any evidence for the clonal expansion of ‘new’ subtypes will continue as part of the NNN program.
Mortality data were assessable in only a proportion of cases and must be interpreted with caution. The NNN does not attempt collection of morbidity data associated with IMD.
A penicillin MIC of 1 mg/L was detected in a single isolate in 2005. NNN trend data show no major shifts in penicillin MICs of invasive strains. Penicillins remain a suitable treatment for IMD in Australia. All isolates were susceptible to the third generation cephalosporins and the prophylactic agents rifampicin and ciprofloxacin retained their high rate of in vitro efficacy.
Top of page
Acknowledgement
Isolates were received in the reference centres from many laboratories throughout Australia. The considerable time and effort involved in forwarding these strains is recognised and these efforts are greatly appreciated. These data could not have been provided without this assistance and the help of clinical colleagues and public health personnel.
The Australian Government Department of Health and Ageing provided a grant for the National Neisseria Network.
Australian Meningococcal Surveillance members, 2005: John Bates, Denise Murphy, Helen Smith, Public Health Microbiology, Queensland Health Scientific Services, Coopers Plains, Queensland; Athena Limnios, Sanghamitra Ray, Nhu Lan Nguyen and John Tapsall, Department of Microbiology, The Prince of Wales Hospital, Randwick, New South Wales; Jo Mercer and Robert Porrit, Department of Microbiology and Infectious Diseases, SWAPS, Liverpool, New South Wales; Julia Griffith, Angelo Zaia, and Geoff Hogg, The Microbiological Diagnostic Unit (PHL, Department of Microbiology and Immunology, University of Melbourne, Parkville, Victoria); Andrew Lawrence, Microbiology Department, Women’s and Children’s Hospital, North Adelaide SA, South Australia; Kathy Bayley and Tony Keil, Department of Microbiology, Princess Margaret Hospital for Children, Subiaco, Western Australia; Mark Gardam and Alistair Macgregor, (Department of Microbiology and Infectious Diseases, Royal Hobart Hospital, Hobart, Tasmania); Gary Lum and Microbiology Staff, (Microbiology Laboratory, Royal Darwin Hospital, Casuarina, Northern Territory); Susan Bradbury and Peter Collignon, (Microbiology Department, Canberra Hospital, Garran, Australian Capital Territory).
Participants in the Australian Meningococcal Surveillance Programme (to whom strains should be referred and enquiries directed) are listed below.
Queensland
John Bates/Denise Murphy/Helen Smith
Public Health Microbiology
Queensland Health Scientific Services
39 Kessels Road
Coopers Plains Qld 4108
Telephone: +61 7 3274 9101
Facsimile : +61 7 3274 9175
Email: john_bates@health.qld.gov.au
Western Australia
Ms K Bayley/Dr ADKeil
Department of Microbiology
Princess Margaret Hospital for Children
1 Thomas Street
Subiaco WA 6008
Telephone: +61 8 9340 8273
Facsimile: +61 8 9380 4474
Email: Kathy.Bayley@health.wa.gov.au
Tasmania
Dr A McGregor/ Mr Mark Gardam
Department of Microbiology and Infectious Diseases
Royal Hobart Hospital
GPO Box 1061L
Hobart Tasmania 7001
Telephone: +61 3 6222 8022
Email: mark.gardam@dchs.tas.gov.au
South Australia
Mr A Lawrence
Microbiology Department
Women’s and Children’s Hospital
72 King William Road
North Adelaide SA 5006
Telephone: +61 8 8161 6376
Facsimile: +61 8 8161 6051
Email: lawrencea@wch.sa.gov.au
Australian Capital Territory
Dr P Collignon/Ms S Bradbury
Microbiology Department
The Canberra Hospital
PO Box 11
Woden ACT 2606
Telephone: +61 2 6244 2425
Email: peter.collignon@act.gov.au
Northern Territory
Dr G Lum and staff
Microbiology Laboratory, NTGPS
Royal Darwin Hospital Campus
Tiwi NT 0810
Telephone: +61 8 8922 8034
Facsimile: +61 8 8980 0714
E-mail: Gary.Lum@nt.gov.au
Victoria
Geoff Hogg
Director
Microbiological Diagnostic Unit Public Health Laboratory (MDU PHL)
Department of Microbiology and Immunology
The University of Melbourne
Parkville Victoria 3052
Telephone: +61 3 8344 5701
Facsimile: +61 3 8344 7833
Email: g.hogg@mdu.unimelb.edu.au
New South Wales
J. Tapsall/A Limnios/TR Shultz
Microbiology Department
SEALS
The Prince of Wales Hospital
Randwick NSW 2031
Telephone: +61 2 9382 9079
Facsimile: +61 2 9398 4275
Email: j.tapsall@unsw.edu.au
J Mercer/R Porrit
Department of Microbiology and Infectious Diseases
SWAPS
Locked Mail Bag 90
Liverpool NSW 2179
Telephone: +61 2 9828 5128
Facsimile: +61 2 9828 5129
Email: Joanne.Mercer@swsahs.nsw.gov.au
Robert.Porritt@swsahs.nsw.gov.au
Top of page
References
1. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2004. Commun Dis Intell 2005;29:149–158.
2. National Neisseria Network. Meningococcal Isolate Surveillance Australia, 1994. Commun Dis Intell 1995;19:286–289.
3. National Neisseria Network. Meningococcal Isolate Surveillance Australia, 1995. Commun Dis Intell 1996;20:422–424.
4. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 1996. Commun Dis Intell 1997;21:217–221.
5. Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal surveillance programme, 1997. Commun Dis Intell 1998;22:205–211.
6. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 1998. Commun Dis Intell 1999;23:317–323.
7. Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 1999. Commun Dis Intell 2000;24:181–189.
8. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2000. Commun Dis Intell 2001;25:113–121.
9. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2001. Commun Dis Intell 2002;26:407–418.
10. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2002. Commun Dis Intell 2003;27:196–208.
11. The Australian Meningococcal Surveillance Programme. Annual report of the Australian Meningococcal Surveillance Programme, 2003. Commun Dis Intell 2004;28:194–206.
12. Australian Gonococcal Surveillance Programme. Penicillin sensitivity of gonococci in Australia: development of an Australian Gonococcal Surveillance Programme. Br J Vener Dis 1984;60:226–230.
13. Porrit RJ, Mercer JL, Munro R. Detection and serogroup determination of Neisseria meningitidis in CSF by polymerase chain reaction (PCR). Pathology 2000;32:42–45.
14. Gray SJ, Borrow R, Kaczmarski EB. Meningococcal serology. In: Pollard AJ, Martin MCJ, eds. Meningococcal disease methods and protocols. Humana Press, Totawa, New Jersey, 2001 pp 61–87.
15. Robertson PW, Reinbott P, Duffy Y, Binotto E, Tapsall JW. Confirmation of invasive meningococcal disease by single point estimation of IgM antibody to outer membrane protein of Neisseria meningitidis. Pathology 2001;33:375–378.
16. Lahra MM, Robertson PW, Whybin R, Tapsall JW. Enhanced serological diagnosis of invasive meningococcal disease by determining anti-group C capsule IgM antibody by EIA. Pathology 2005;37:239–241.
17. Bryant PA, Hua YL, Zaia A, Griffith J, Hogg G, Curtis N, et al. Prospective study of a Real-Time PCR that is highly sensitive, specific and clinically useful for diagnosis of meningococcal disease in children. J Clin Microbiol 2004;42:2919–2925.
Top of page
Author affiliations
Corresponding author: Associate Professor John Tapsall, Department of Microbiology, SEALS, The Prince of Wales Hospital, High Street, Randwick, NSW 2031
This report was published in Communicable Diseases Intelligence Vol 30 No 2, June 2006.
