The Australian Group for Antimicrobial Resistance (AGAR) has played a unique role in surveillance of antimicrobial resistance in Australia. It has a broad laboratory membership representing the major teaching hospitals in all Australian capitals and more recently major private pathology laboratories in most states. The use of an active surveillance strategy with standard methodology for collection and examination of clinically significant isolates has produced data accurately reflecting the changing prevalence of antimicrobial resistance in major hospitals as well as the community. AGAR has documented the spread of methicillin-resistant Staphylococcus aureus in Australian hospitals in the late 1980s and throughout the 1990s. Surveys of antimicrobial resistance in enterococci have monitored the emergence of vancomycin-resistant enterococci as an important nosocomial pathogen in Australia. AGAR has also conducted major national surveys of resistance in Streptococcus pneumoniae, community isolates of Staphylococcus aureus, Haemophilus influenzae and in the Enterobacteriaceae. These and other activities have given AGAR a unique perspective on emerging patterns of resistance in key pathogens in Australia. The recent extension of membership to include more private pathology laboratories may provide the opportunity to conduct more representative community based surveys. Commun Dis Intell 2003;27 Suppl:S47-S54.
Top of page
Introduction
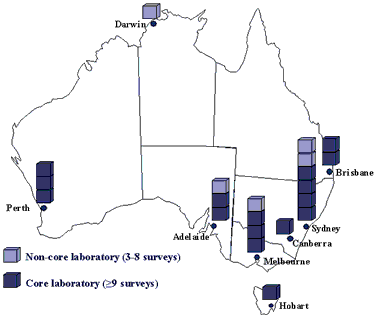
The Australian Group for Antimicrobial Resistance (AGAR) has conducted surveillance of antimicrobial resistance in Australian teaching hospitals since 1986. AGAR membership has always includes very broad representation of the major teaching hospitals in all Australian capitals (Figure 1). The spread of methicillin-resistant Staphylococcus aureus (MRSA) was a major concern in the 1980s and surveillance of hospital staphylococcal infections was the first activity of AGAR. Periodic surveys of the prevalence of resistance in hospital isolates of S. aureus have now been conducted for 15 years.1,2 This represents the most complete prospective national description of the evolution of resistance in hospital isolates of S. aureus.
Figure 1. Distribution of participating teaching hospital laboratories in capital cities
The emergence of resistance in other groups of bacteria has prompted AGAR to conduct a number of organism specific surveys. An extensive study of resistance in Haemophilus influenzae was conducted prior to the introduction of vaccination for H. influenzae type b.3 The emergence of antimicrobial resistance in Streptococcus pneumoniae has caused worldwide concern and AGAR has been able to demonstrate over four surveys the trend towards multiple antimicrobial resistance and penicillin resistance in pneumococci in Australia.4,5 Ongoing surveys of antimicrobial resistance in enterococci have also been conducted in response to increasing interest in vancomycin-resistant enterococci. Changing patterns of resistance in the Enterobacteriaceae and the emergence of extended-spectrum ▀-lactamases (ESBL) led to surveys of resistance trends in Escherichia coli and Klebsiella pneumoniae.
Top of page
Methods
The AGAR surveys have been conducted prospectively with standardised methods for data collection, isolate collection and laboratory examination. Collections consisting of unique clinically significant sequential isolates, excluding duplicates and screening isolates, have been stored for future reference. Methods for identified, susceptibility testing and typing have been described in detail elsewhere.1,2,3,4,5 Standard quality control organisms were included in each susceptibility testing batch with results validated by a coordinating laboratory.
Top of page
Results
Resistance in Staphylococcus aureus
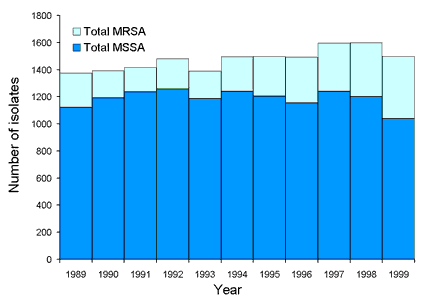
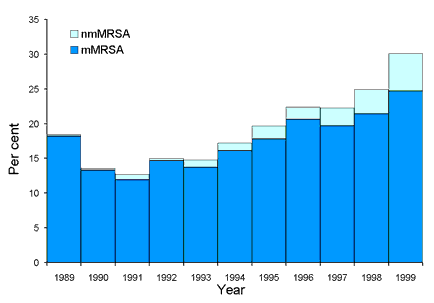
The first three surveys of the prevalence of resistance in teaching hospital isolates of S. aureus were conducted during 1986 and 1987.1 This first national study involved 14 teaching hospital laboratories in the national capital and all mainland state capitals. The study established that the prevalence of MRSA as a proportion of clinically significant isolates of S. aureus was 14.4 per cent. Annual surveys were conducted from 1989 to 1999 and the results have been published2 or submitted for publication. The total number of isolates collected in each year and the number of MRSA isolates are shown in Figure 2. In the initial study most MRSA isolates were multiresistant. In the subsequent annual surveys MRSA was divided into two groups based on resistance to erythromycin, tetracycline, trimethoprim, gentamicin, rifampicin, fusidic acid, ciprofloxacin and mupirocin. Isolates which were resistant to three or more of the above were defined as multiresistant (mMRSA), and those resistant to less than three as non-multiresistant (nmMRSA). Data collected by AGAR and others showed that this latter non-multiresistant group appeared to arise more often in the community setting.6,7,8 The trends in prevalence of mMRSA and nmMRSA are shown in Figure 3. Both show marked and sustained increases in the late 1990s.
Figure 2. Number of MRSA and MSSA isolates collected from 1989 to 1999
Top of page
Figure 3. mMRSA and nmMRSA isolates collected from 1989 to 1999 as a proportion of all Staphylococcus aureus isolates
Resistance in Haemophilus influenzae
Thirty-four laboratories were involved in the collection of 970 isolates of H. influenzae between 1988 and 1990.3 Minimal Inhibitory Concentrations (MICs) to 16 different antibiotics were determined. The overall rate of beta-lactamase production was 16 per cent but there was wide variation between the states. In Adelaide the rate was 4.5 per cent and in Canberra 28.6 per cent. There was also a marked difference between invasive strains (e.g., blood) and non-invasive strains (e.g., sputum). In invasive strains beta-lactamase production was 22.3 per cent but in respiratory tract isolates it was 35.3 per cent. Most strains were resistant to erythromycin. Variable resistance was seen to most other antibiotics with geographical differences as well as differences between invasive and non-invasive strains. In non-invasive strains the resistance to amoxicillin-clavulanate, chloramphenicol, tetracycline, trimethoprim and co-trimoxazole were 2.1 per cent, 1.8 per cent, 4.5 per cent, 12.2 per cent and 5.7 per cent respectively.
Top of page
Resistance in Streptococcus pneumoniae
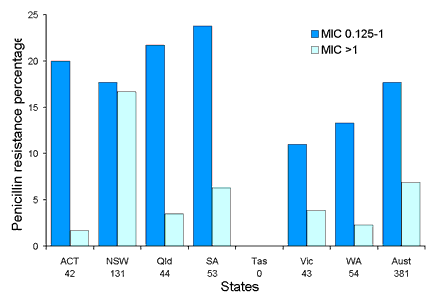
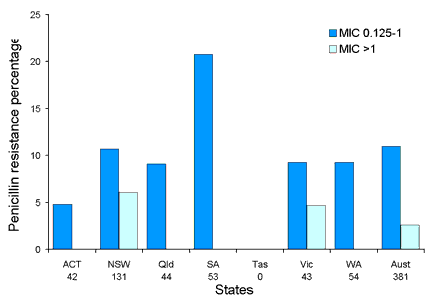
Antimicrobial resistance in Streptococcus pneumoniae has been monitored by the AGAR group in three survey periods, 1989, 1994 and 1999. Up to 100 consecutive clinically significant isolates were collected from each participating laboratory. All isolates collected for the 1994 and 1999 surveys had a penicillin MIC determined using Etest strips (AB Biodisk, Solna, Sweden). Erythromycin, tetracycline, chloramphenicol and co-trimoxazole were tested using disc diffusion. Penicillin resistance increased significantly between 1989 and 1994, however, this trend levelled out in 1999. Overall 86 per cent of invasive isolates and 75 per cent of non-invasive isolates were penicillin susceptible (MIC < 0.064 mg/L). High-level penicillin resistance (MIC > 1 mg/L) was found in 2.6 per cent of invasive isolates and 6.9 per cent of non-invasive isolates. Prevalence of penicillin resistance in invasive and non-invasive isolates in each state is shown in Figures 4 and 5. Multi-drug resistant strains were common with over 8 per cent resistant to three or more antimicrobial agents tested in 1994. In 1999, 6.8 per cent of invasive and 16.7 per cent of non-invasive isolates were multi-drug resistant. Another survey was conducted in 2002.
Figure 4. Proportion of penicillin resistance in Streptococcus pneumoniae in invasive isolates by state, 1999
Top of page
Figure 5. Proportion of penicillin resistance in Streptococcus pneumoniae in non-invasive isolates by state, 1999
Resistance in enterococci
Two surveys to study the prevalence of antimicrobial resistance in Enterococcus spp. in hospitals were conducted, one in 1995 and another in 1999. For each study period, up to 100 consecutive clinically significant strains were identification and antimicrobial susceptibility tests performed using the participating institutions routine methods. Any strain demonstrating resistance to ampicillin, vancomycin or high level aminoglycoside resistance, and all non E. faecalis isolates were referred to a central testing laboratory for confirmation. Correct identification of enterococci to species level was problematic. The proportion of E. faecium increased from 5 per cent of all isolates in 1995 to nearly 10 per cent in 1999. No vancomycin resistance was detected in the first study period, however, in 1999, six (0.3%) strains were resistant. Ampicillin resistance in E. faecium increased from 57 per cent in 1995 to 77 per cent in 1999. Beta-lactamase production remains uncommon, with only one ▀-lactamase producing E. faecalis detected.
During 1999, 18 institutions collected all enterococci isolated from blood cultures. Over 370 strains were isolated, of which 74 per cent were E. faecalis, and 20 per cent were E. faecium. Vancomycin-resistance was detected in 8 per cent of all E. faecium isolates. All were vanB and were from three institutions.
Top of page
Resistance in Escherichia coli and Klebsiella spp.
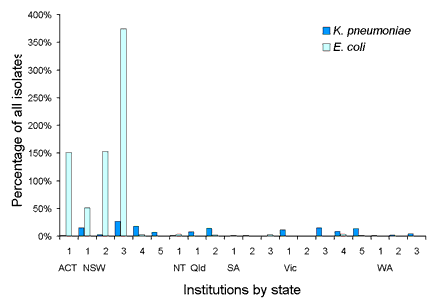
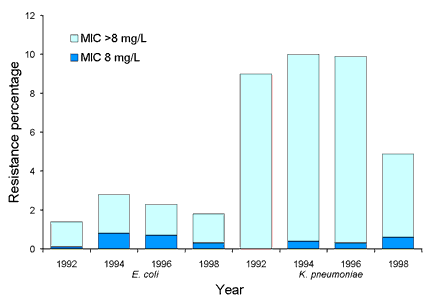
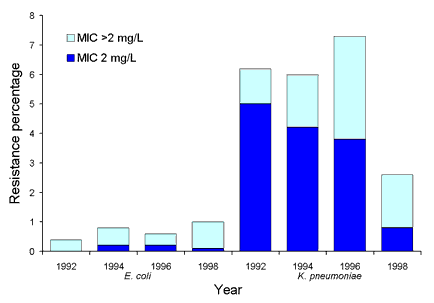
Surveys of resistance and multi-resistance in Escherichia coli and Klebsiella spp. have been conducted biannually since 1992. Up to 50 isolates from each species were collected from 14-20 institutions throughout Australia. MICs to 14 antimicrobial agents (from 8 drug classes) were determined using a customised conventional MicroScan broth microdilution panel (Dade Behring, West Sacramento, California). In E. coli ampicillin resistance has been prevalent since the beginning of surveillance at approximately 50 per cent, while non-susceptibility to amoxicillin-clavulanate has remained at under 10 per cent. Multi-resistant E. coli (resistance to 4 or more drug classes) were uncommon (<4%). K. pneumoniae with an extended-spectrum ▀-lactamase phenotype were prevalent in some institutions (Figure 6). Overall 8 per cent (range 0-26%) of K. pneumoniae were ceftazidime resistant (MIC > 1 mg/L). While most institutions demonstrated a decline in the number of K. pneumoniae with ESBL phenotypes since 1992, others have shown a steady increase since 1996. The number of E. coli that were ceftazidime resistant has remained steady at less than 1.5 per cent since 1992 (Figure 7). Between 1992 and 1998, the rate of ceftazidime resistant K. pneumoniae varied between 6-10 per cent with an increasing proportion of isolates with an MIC > 8 mg/L (Figure 7). Gentamicin resistance was also common in some institutions and closely paralleled ESBLs (Figure 8). Ciprofloxacin non-susceptibility (MIC > 1 mg/L) was uncommon in E. coli (< 1%). Ciprofloxacin non-susceptible K. pneumoniae decreased from 6-7 per cent in 1992-1996 to less than 3 per cent in 1998 (Figure 9). No carbapenem resistance has been detected. An increase in co-trimoxazole resistance (MIC > 8 mg/L) was observed between 1992 and 1994 (11% to 19%), but the level of resistance has since remained steady at approximately 20 per cent (data not shown).
Figure 6. Extended-spectrum ▀-lactamase phenotype in Escherichia coli and Klebsiella pneumoniae in 20 institutions, 1992 to 1998
Top of page
Figure 7. Proportion of ceftazidime resistance in Escherichia coli and Klebsiella pneumoniae, 1992 to 1998
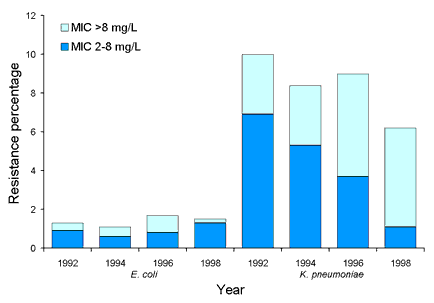
Figure 8. Proportion of gentamicin resistance in Escherichia coli and Klebsiella pneumoniae, 1992 to 1998
Top of page
Figure 9. Proportion of ciprofloxacin resistance in Escherichia coli and Klebsiella pneumoniae, 1992 to 1998
Future directions
AGAR will continue to publish surveys of antimicrobial resistance in bacteria causing serious health problems within Australia. Organisms of interest will include Staphylococcus aureus, pneumococcus, Enterococcus spp, E. coli, Klebsiella spp. and Enterobacter spp. As significant problems emerge in other organisms they will also be subject to survey where practicable. AGAR may also test organisms other than bacteria where there is currently little or no data but potential for antimicrobial resistance to compromise clinical care (e.g., fungi).
Additional major private laboratories have joined AGAR recently so that private pathology is now represented in each mainland state. This has given AGAR the ability to conduct valid surveys of community-acquired as well as health care-associated infections. Opportunities also exist for closer liaison with other groups involved in surveillance of health care-associated infections (e.g., with Australian Infection Control Association on national surveillance of wound and blood stream infections). A collaborative approach should provide better quality national data on all aspects of infection including antimicrobial resistance. AGAR surveys to date have mainly been limited to major teaching hospitals in capital cities. While we believe those results reflect the situation in those institutions, we have not attempted any population based surveys. Recent consolidation of pathology service provision and the availability of more flexible information technology may both serve to make such surveys a more practical possibility. Matching of data from such surveys to other information such as antimicrobial consumption may also be possible in future creating a powerful tool for monitoring the effectiveness of initiatives aimed at encouraging rational prescribing.
Top of page
Continued funding of AGAR is a vexed issue. While funding for the survey activities of AGAR has been primarily by the participating laboratories, one major pharmaceutical company sponsored biannual meetings of participants from 1986 to May 2002. AGAR must now identify stable ongoing sources of funds to enable it to continue its current program and where appropriate broaden the scope of its activities, for example in the area of community-acquired infection. Whether funding can be sourced from the pharmaceutical industry or from government remains to be seen. The former possibility may be problematic given the reduction in development and marketing of antimicrobials in recent years.
For 15 years AGAR has provided data on the evolution of antimicrobial resistance in major pathogens in Australia. In the future it is hoped AGAR will not only continue this important task but also broaden the range of its surveys to cover emerging resistance in all organisms of importance in community and health care-associated infection. Efforts need to be made to ensure sampling is representative of hospital and community trends, to correlate results with other relevant data and to disseminate results widely in a more timely manner.
Top of page
Acknowledgement
Eli Lilly Australia Pty Ltd was the major sponsor of AGAR from 1986 to 2002. We also acknowledge the significant contribution by Mrs Pam Catanach.
Top of page
References
1. Turnidge J, Lawson P, Munro R, Benn R. A national survey of antimicrobial resistance in Staphylococcus aureus in Australian teaching hospitals. Med J Aust 1989;150:65-72.
2. Turnidge JD, Nimmo GR, Francis G. Evolution of resistance in Staphylococcus aureus in Australian teaching hospitals. Med J Aust 1996;164:68-71.
3. Collignon P, Bell J, MacInnes S, Gilbert G, Toohey M. A national collaborative study of resistance to antimicrobial agents in Haemophilus influenzae in Australian hospitals. J Antimicrob Chemother 1992;30:153-163.
4. Collignon P, Bell J. Streptococcus pneumoniae: How common is penicillin resistance in Australia? Aust N Z J Med 1992;22:473.
5. Collignon P, Bell J. Drug-resistant Streptococcus pneumoniae: The beginning of the end for many antibiotics? Med J Aust 1996;164:64-67.
6. Collignon P, Gosbell I, Vickery A, Nimmo G, Stylianopoulos T, Gottlieb T. Community-acquired methicillin-resistant Staphylococcus aureus in Australia. Lancet 1998;352:146-147.
7. Nimmo GR, Schooneveldt J, O'Kane G, McCall B, Vickery A. Community acquisition of gentamicin-sensitive MRSA in south-east Queensland. J Clin Microbiol 2000;38:3926-3931.
8. Gosbell IB, Mercer JL, Neville SA, Crone SA, Chant KG, Jalaludin BB, et al. Non-multiresistant and multi-resistant methicillin-resistant Staphylococcus aureus in community-acquired infections. Med J Aust 2001;174:627-630.
Top of page
Author affiliations
1. Director of Microbiology, Queensland Health Pathology Service, Brisbane, Queensland.
2. Senior Scientist, Microbiology and Infectious Diseases, Women's and Children's Hospital, North Adelaide, South Australia.
3. Director of Microbiology and Infectious Diseases, Canberra Hospital, Garran, Australian Capital Territory
Corresponding author: Dr Graeme Nimmo, Director of Microbiology, Queensland Health Pathology Service, c/-Princess Alexandra Hospital, Brisbane QLD 4102. Telephone: +61 7 3240 2389. Facsimile: +61 7 3240 5786. Email: Graeme_Nimmo@health.qld.gov.au
The Australian Group for Antimicrobial Resistance is (in alphabetical order): John Andrew (Gribbles Pathology Victoria Pty Ltd, Victoria), Jan Bell (Women's and Children's Hospital, South Australia), Richard Benn, Sue Benson (St John of God Pathology, Western Australia), Susan Bradbury (Canberra Hospital, Australian Capital Territory), Keryn Christiansen, Peter Collignon, Geoff Coombs (Royal Perth Hospital, Western Australia), Marion Easton, Joan Faoagali, Clarence Fernandes (Royal North Shore Hospital, New South Wales), Graham Francis (Fremantle Hospital, Western Australia), Glenn Funnell (Concord Repatriation General Hospital, New South Wales), Sue Garland, Narelle George (QHPS, Royal Brisbane Hospital, Queensland), Gena Gonis, Iain Gosbell, Tom Gottlieb, Jacqueline Harper, Linda Joyce (St Vincent's Hospital, Victoria), PC Lee (Gribbles Pathology, South Australia), Irene Lim, Gary Lum (Royal Darwin Hospital, Northern Territory), David McGechie, Alistair McGregor (Royal Hobart Hospital, Tasmania), David Mitchell (Westmead Hospital, New South Wales), Leigh Mulgrave (PathCentre, Western Australia), Stephen Neville (South Western Area Pathology Service, New South Wales), Graeme Nimmo, Miriam Paul (Douglas Henley Moir Pathology, New South Wales), John Pearman, Hendrik Pruul (Flinders Medical Centre, South Australia), Jenny Robson (Sullivan Nicolaides Pathology, Queensland), David Rose (Nepean Hospital, New South Wales), Jacqueline Schooneveldt (QHPS, Princess Alexandra Hospital, Queensland), Denis Spelman (Clare Franklin Alfred Hospital, Victoria), Joanne Stylianopoulos, Anastasia Stylianopoulos (Royal Children's and Women's Hospitals, Victoria), John Turnidge, Alison Vickery, Mary Jo Waters, Bruce Winter (Institute of Medical and Veterinary Science, South Australia), Barbara Yan (Royal Prince Alfred Hospital, New South Wales).
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
