Campylobacter is a common cause of bacterial gastroenteritis in Australia. Antibiotic resistance among Campylobacter is an emerging problem in Europe and the United States of America. Monitoring may detect emerging resistance. Since there is no epidemiologically validated subtyping system for Campylobacter, antimicrobial resistance patterns may prove useful as an epidemiological marker. Campylobacter isolates from residents of the Hunter region were differentiated by PCR into two categories: C. jejuni and non-C. jejuni. Minimal inhibitory concentrations (MIC) were determined for 10 antibiotics using the National Committee for Clinical Laboratory Standards (NCCLS) agar dilution methodology. Risk factor information including travel history were obtained as part of a case-control study by conducting telephone interviews with infected individuals. Sixty-four per cent, 3.4 per cent, 3.4 per cent and 11.2 per cent of C. jejuni isolates were resistant to ampicillin (at MIC > 8 mg/L), erythromycin (> 8 mg/L), nalidixic acid (> 32 mg/L) and tetracycline (> 8 mg/L), respectively. A diverse pattern of antibiotic resistance ('resistotypes') was detected with some change occurring over time. Several possible clusters of Campylobacter infections were identified based on resistotype. Of seven infections acquired during overseas travel, 57 per cent (4/7) were resistant to more than one antibiotic class compared to 10 per cent (14/144) of locally-acquired isolates (p=0.004, Fisher exact). The potential usefulness of resistotyping as an epidemiological marker is worthy of further exploration. Commun Dis Intell 2003;27 Suppl:S80-S88.
Top of page
Introduction
Campylobacter is the most common bacterial cause of foodborne disease in Australia. More than 15,000 cases of Campylobacter infection are reported in Australia each year, excluding New South Wales where the disease is not notifiable (Communicable Diseases Network Australia -National Notifiable Diseases Surveillance System, personal communication). Antibiotic therapy is generally not recommended for the treatment of campylobacteriosis, however, antimicrobials are prescribed at times (Hunter Public Health Unit, 2002, unpublished data) and therapy is warranted in some circumstances.1
Antimicrobial resistance among Campylobacter isolates was first observed in the early 1990s.2 Resistance among Campylobacter isolates has been reported from the United States of America (USA),3 Europe,4,5,6 the United Kingdom,7,8 Asia,9,10 the Middle East11 and Australia.12 In particular, resistance to quinolones has been widely observed.4,5,6,13,14 Recently, reports describing increasing prevalence of quinolone resistances have been made in the Netherlands,6 the USA3 and the United Kingdom.7,8 However, resistance to macrolides such as erythromycin remains low among isolates from humans9 and animals.5
There have been at least two previous surveys of Campylobacter resistance in Australia. Huysman and Turnidge15 examined 79 clinical strains of C. jejuni from South Australian patients isolated prior to 1997. Riley (personal communication T. Riley, University of Western Australia, 2001) examined 50 clinical and 50 environmental strains of Campylobacter species isolated in Western Australia between 1999 and 2000. These studies were descriptive only and did not explore the use of resistance typing as an epidemiological marker.
Antibiotics are used in the livestock industry and it has been suggested that their use in food animals has contributed to the development of antibiotic resistance in human isolates. Increases in the detection of quinolone resistant Campylobacter were reported from the UK after licensing of enrofloxacin for veterinary use16 and experimental evidence suggests that the use of quinolones in broiler chickens leads to the selection of resistant Campylobacter organisms.17 In Australia, legislation limits the use of particular antibiotics such as fluoroquinolones, cephalosporins, gentamicin, and chloramphenicol in food producing animals.18 However, use of these agents is widespread in some countries and therefore imported foods may be a source of resistant organisms.
With the global concern with the increasing prevalence of resistance among clinically important bacterial pathogens, the Joint Expert Technical Advisory Committee on Antibiotic Resistance (JETACAR) examined the use of antibiotics in food producing animals and its association with emergence of resistance. The JETACAR report made several recommendations for regulatory control of antibiotic use, monitoring and surveillance of resistance in clinical isolates, strategies for antibiotic use, infection prevention and hygiene, education, research, communication and coordination of resistance management programs.18
A study of antibiotic resistance among Campylobacter isolates from residents of the Hunter region was initiated as part of monitoring and surveillance efforts. This report describes antibiotic resistance profiles of human Campylobacter isolates and is part of an evaluation of multiple typing methods for their usefulness to examine specific risk factors for Campylobacter infection.
Top of page
Methods
Epidemiological methods
A case control study was conducted in the Hunter region of New South Wales, which has a population of 570,000 and includes urban, rural and semi-rural areas. Cases were recruited using voluntary notifications from two participating laboratories of the three major pathology service providers for the population. A total of 355 cases were enrolled between January 1999 and July 2001. Telephone interviews were conducted after verbal consent was given and information on illness, travel, foods consumed, dining locations, drinking and recreational water sources, animal contact and demographics was obtained.
Isolates
Of the 355 enrolled cases, 240 Campylobacter isolates were detected at the public laboratory (Hunter Area Pathology Service, HAPS). Of these, 171 stored isolates were available for inclusion in this study. An additional 29 Hunter isolates obtained between July to September 2001 from patients not enrolled in the case control study were included to bring the total tested up to 200.
Laboratory methods
Diarrhoeal stool was cultured on charcoal blood-free agar with cefoperazone and amphotericin B (Biomerieux). Plates were incubated microaerobically at 42°C for 48 hrs. Campylobacter species were motile isolates with characteristic gram stain appearance and oxidase positivity. Isolates were stored at -70°C in glycerol broth until analysed.
Speciation of each isolate was determined by hippurate hydrolysis and polymerase chain reaction (PCR) targeted at C. jejuni specific hippuricase and putative oxidoreductase genes as described previously.19,20,21 There was complete concordance between tested hippurate and PCR species status. Template DNA for PCR was prepared using Instagene matrix as outlined in the manufacturer's instructions (BioRad, California, USA). PCR amplifications were performed by previously described methods19,20,21 and the amplification products were analysed on 1 per cent agarose gels. This enabled one classification of the isolates as either C. jejuni or non-C. jejuni.
Susceptibility testing was performed by agar dilution methodology utilising Mueller-Hinton agar with 5 per cent lysed sheep blood in accord with National Committee for Clinical Laboratory Standards (NCCLS) methodology for Helicobacter species.22 The inoculum was prepared as a saline suspension equivalent to a 2.0 McFarland standard from a 48-hour blood agar subculture and inoculated with a replicator machine. This technique places 1 µl of suspension per spot onto the agar dilution medium. Media were prepared containing doubling dilutions through a full range of concentrations for quinolone agents (nalidixic acid, norfloxacin and ciprofloxacin), tetracycline, ampicillin, gentamicin and macrolide agents (erythromycin, azithromycin, clarithromycin, roxithromycin). The inoculated plates were incubated microaerobically for 48 hours. The Minimal Inhibitory Concentration (MIC) was defined as the lowest concentration giving complete inhibition of visible growth on the plate. Interpretation of MIC levels were made with reference to accepted breakpoint values where available. E. coli ATCC 25922 and S. aureus ATCC 29213 were used as quality control strains. The MIC50 and MIC90 values for each antibiotic were calculated from the distribution of the MIC values of all isolates. MIC50 values represent the concentration of antibiotic below which growth of 50 per cent of isolates were inhibited and MIC90 value represent the concentration of antibiotic below which growth of 90 per cent of isolates were inhibited.
Statistical methods
Comparison of the proportions was performed using the Fisher exact test or Yates corrected χ2 test as appropriate and comparison of proportion of resistance over time was performed using χ2 for trend using Epi Info version 6.04c.
Top of page
Results
The isolates included in this report comprised those stored at one of the two participating laboratories. HAPS is the public laboratory for the Hunter Health Area, and services a different population from many of the private laboratories. Of the 240 HAPS-identified cases that participated in the case control study, 90 (37.5%) were admitted to hospital compared to three (3%) hospitalised cases of the 115 identified through the other pathology service provider (Yates corrected ã2=47.2, p<0.001). Thus, cases of C. jejuni included in this study probably represent more severe cases.
PCR analysis confirmed 180 of the 200 isolates to be C. jejuni (151 of which were enrolled in the case control study) and only the susceptibility results for the C. jejuni isolates are described in this paper.
MIC50 and MIC90 values for each antibiotic are shown in Table 1 together with resistance levels for those agents with accepted breakpoint values. Ampicillin resistance was common (64%) with tetracycline resistance at 11 per cent. Levels of erythromycin and quinolone resistance were low. Sixty-eight per cent of locally acquired isolates were resistant to at least one class of antibiotic.
Table 1. Resistance of Campylobacter jejuni isolates in Hunter region (n =180)
| |
MIC50 mg/L |
MIC90 mg/L |
Breakpoint |
Resistant
% |
| All Isolates |
Locally-acquired |
Overseas-acquired† |
| Nalidixic acid |
4 |
8 |
> 32 mg/L |
3.4 |
1.4 |
43* |
| Norfloxacin |
0.5 |
1.0 |
|
|
|
|
| Ciprofloxacin |
0.25 |
0.5 |
> 4 mg/L |
2.9 |
0 |
43* |
| Tetracycline |
0.25 |
8 |
> 8 mg/L |
11 |
10 |
43* |
| Ampicillin |
8 |
64 |
> 8 mg/L |
64 |
66 |
57 |
| Erythromycin |
1 |
2 |
> 8 mg/L |
3.4 |
3 |
0 |
| Azithromycin |
0.125 |
0.25 |
|
|
|
|
| Clarithromycin |
1 |
4 |
|
|
|
|
| Roxithromycin |
4 |
16 |
> 8 mg/L |
48 |
40 |
57 |
| Gentamicin |
0.5 |
1.0 |
> 8 mg/L |
0 |
0 |
0 |
| Resistance >1 class |
|
|
|
12 |
9 |
43* |
| Total number of isolates |
|
|
|
n=180† |
n=148 |
n=7 |
Top of page
Figures 1a and 1b show the MIC distributions for macrolide and quinolone antibiotics respectively. The MIC distribution curves were bimodal with a small outlying peak made up of high-level resistant isolates. For macrolides, the isolates were most susceptible to azithromycin and least susceptible to roxithromycin. All the erythromycin resistant isolates were pan-resistant to other macrolides.
Figure 1a. Macrolide MIC distribution for C. jejuni isolates (n=180)
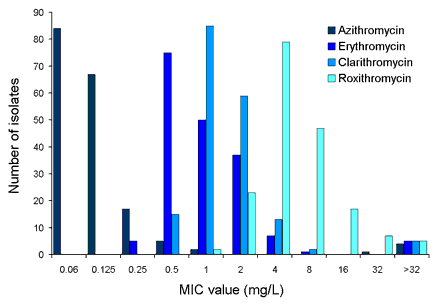
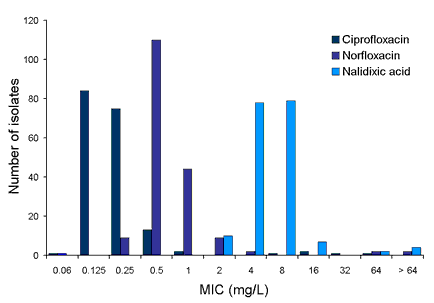
Figure 1b. Quinolone MIC distribution for C. jejuni isolates (n=180)
Top of page
For quinolones, isolates were most susceptible to ciprofloxacin and least susceptible to nalidixic acid. Two isolates with high-level ciprofloxacin resistance were identified (MIC > 32 mg/L), one acquired overseas, one where it was not known whether it was acquired locally or overseas. Two nalidixic acid resistant isolates (both locally-acquired) were susceptible to ciprofloxacin and norfloxacin implying a different resistance genotype.
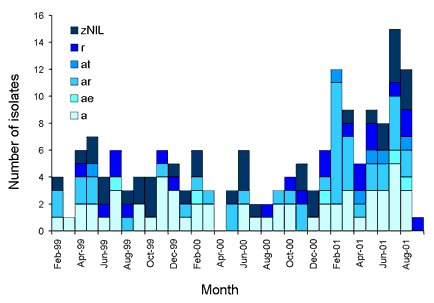
Table 2 shows the variation in resistance patterns ('resistotypes') that occurred over the three years of this study. Ampicillin resistance increased from 59 per cent in 1999 to 69 per cent in 2001 and roxithromycin resistance from 36 to 51 per cent over the same time, however these trends were not statistically significant. Temporal distribution of resistotypes was examined by year (Table 2) and month (Figure 2). There were 15 unique resistance patterns seen among the 180 isolates. Three dominant resistotypes occurred throughout the years of the study. Resistance to ampicillin, roxithromycin, and ampicillin-roxithromycin was observed in each year. Many resistotypes were found over the study period. Ampicillin-tetracycline resistance was found in May 1999 and then again from February 2001 until the end of the study period (Figure 2).
Table 2. Annual distribution of C. jejuni resistotypes, 1999 to 2001
| Resistance pattern (resistotype) |
Count of resistance patterns by year |
Total |
| 1999 |
2000 |
2001 |
| a |
20 |
14 |
21 |
55 |
| act |
|
|
2 |
2 |
| ae |
1 |
1 |
3 |
5 |
| aet |
1 |
|
|
1 |
| ant |
|
1 |
1 |
2 |
| ar |
8 |
10 |
23 |
41 |
| arc |
|
|
1 |
1 |
| art |
2 |
|
2 |
4 |
| at |
1 |
|
4 |
5 |
| ct |
1 |
|
|
1 |
| r |
7 |
3 |
11 |
21 |
| rc |
|
|
1 |
1 |
| rt |
1 |
|
1 |
2 |
| t |
1 |
|
2 |
3 |
| Nil |
13 |
12 |
11 |
36 |
| Total |
56 |
41 |
83 |
180 |
Top of page
Figure 2. Temporal distribution of the major resistotypes, February 1999 to December 2001
Top of page
Four potential clusters of Campylobacter infection were identified on the basis of resistotype. Among the resistotypes of more than 10 isolates (ampicillin (a) resistance, ampicillin-roxithromycin (ar) resistance, and roxithromycin (r) resistance), a cluster was defined as more than two-times the average number of cases in a one month period. One cluster of ampicillin resistant isolates was detected in November to December 1999 (n=7), one from January to March 2001 (n=7) and one in July to August 2001 (n=7). A further cluster of ampicillin-roxithromycin resistance was detected in February to March 2001(n=13) (Figure 2).
All seven isolates acquired during overseas travel were resistant to at least one class of antibiotic. There were two nalidixic acid (one with coincident ciprofloxacin resistance) and three tetracycline resistant isolates. Fifty-seven per cent (4/7) of overseas-acquired isolates were resistant to more than one antibiotic class compared to 10 per cent (14/144) of locally-acquired isolates (p=0.004, Fisher exact). Isolates acquired overseas had similar levels of ampicillin resistance to locally acquired isolates. Quinolone and tetracycline resistance were significantly more frequent in overseas isolates (Table 1).
Eight per cent (12/150) of the patients took antibiotic therapy in the month prior to Campylobacter infection. The resistance rates among those exposed to antibiotics (11/12, 92%) was higher compared to unexposed subjects (109/138, 79%; Odds ratio 2.95, 95% CI 0.37-23.8, p=0.46). This was not statistically significant, possibly due to the low power to detect a difference, limited by the small sample size.
Top of page
Discussion
Patterns of antibiotic resistance among the isolates included in this study were similar to previous studies conducted in Australia. A prevalence of ampicillin resistance of 64 per cent (MIC >8) was similar to the levels seen in South Australia16 and Spanish paediatric isolates.23 The majority of this resistance is due to ß-lactamase production in that the majority of resistance is abolished by the addition of clavulanate. In the latter study, amoxycillin/clavulanate resistance began to emerge implying an alternative resistance mechanism.
Seven isolates with quinolone resistance were detected in the current study. Quinolone resistance was more common in the isolates acquired overseas. Binotto et al12 described two cases of quinolone resistant Campylobacter infection in travellers returning to Australia from the United Kingdom. Riley found 4 of 50 (8%) Western Australian clinical strains had ciprofloxacin resistance (MIC >4) (personal communication T. Riley, University of Western Australia, 2001). Huysman et al found no quinolone resistance in isolates from South Australia.15
The usual evolution of quinolone resistance involves mutations in the quinolone resistance-determining region of the gyrA (topoisomerase II) gene.24 Initial mutations produce high-level nalidixic acid resistance, with additional changes leading to increasing ciprofloxacin resistance. Active multi-drug efflux mechanisms for quinolone resistance in Campylobacter are also described25 and may be responsible for reducing susceptibility to quinolones, ß-lactams, tetracycline, chloramphenicol and other agents.26 The molecular mechanisms of resistance in the present study's isolates are to be confirmed through further study.
Levels of erythromycin resistance were low in the present study (3.4%) as previously described in Australia16 and other countries.9 None of the overseas-acquired isolates in the current study showed macrolide resistance. Roxithromycin resistance (48%) was more prevalent and therefore provided a more useful contribution to resistotype diversity than erythromycin.
Gentamicin resistance was not detected among these isolates similar to findings from the other Australian studies. Studies of Campylobacter gentamicin susceptibility overseas have mostly shown universal susceptibility.7,27 However, Reina et al,23 documented 12 resistant isolates (2.2%) in the last two years (1992-93) of that study.
In addition to the descriptive epidemiology of resistotypes, antibiotic resistance was explored for its potential usefulness as an epidemiological marker. Outbreaks of campylobacteriosis are rarely detected, due to the inability to recognise the existence of a cluster by serotype or phage type distributions. The small clusters of resistotypes that were observed in this study possibly represented outbreaks. Furthermore, a temporal trend was detected for one pattern (ampicillin-tetracycline resistance). The diversity of antibiotic resistance found among these isolates suggests that resistance patterns may be useful as an epidemiological marker. Resistotyping is being evaluated for its epidemiological value in comparison with eight Campylobacter subtyping methods. Comparison of the major resistotypes with respect to range of food, water and environmental exposures for infection from this case set will also be undertaken. The difference in resistance between overseas and locally acquired isolates in these human isolates further supports its potential use as an epidemiological marker.
The findings outlined in this report suggest that routine antibiotic resistance testing for Campylobacter may prove useful to assess emerging resistance and to detect clusters. If resistotyping is to be performed on a routine basis, standardised testing protocols will need to be developed.
Top of page
Acknowledgements
This study was funded by the OzFoodNet enhanced surveillance program of the Department of Health and Ageing, Australia.
Top of page
References
1. Chin J ed. Control of Communicable Diseases Manual, 2000, 17th edition. American Public Health Association.
2. Allos BM. Campylobacter jejuni infections: update on emerging issues and trends. Clin Infect Dis 2001;32:1201-1206.
3. Smith KE, Besser JM, Hedberg CW, Leano FT, Bender JB et al. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N Engl J Med 1999;340:1525-1532.
4. Sjogren E, Lindblom G-B, Kaijser B. Norfloxacin resistance in Campylobacter jejuni and Campylobacter coli isolates from Swedish patients. J Antimicrob Chemother 1997;40:257-261.
5. Saenz Y, Zarazaga M, Lantero M, Gastanares MJ, Baquero F, Torres C. Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997-1998. J Antimicrob Chemother 2000;44:267-271.
6. Talsma E, Goettsch WG, Nieste HL, Schrijnemakers PM, Sprenger MJ. Resistance in Campylobacter species: increased resistance to fluoroquinolones and seasonal variation. Clin Infect Dis 1999;29:845-848.
7. Thwaites RT, Frost JA. Drug resistance in Campylobacter jejuni, C. coli and C. lari isolated from humans in north west England and Wales, 1997. J Clin Pathol 1999;52:812-814.
8. Sam WIC, Lyons MM, Waghorn DJ. Increasing rates of ciprofloxacin resistant Campylobacter. J Clin Pathol 1999;52:709-710.
9. Isenbarger DW, Hoge CW, Srijan A, Pitarangsi C, Vithayasai N, Bodhidatta L, et al. Comparative antibiotic resistance of diarrheal pathogens from Vietnam and Thailand, 1996-1999. Emerg Infect Dis 2002;8:175-180.
10. Ananthan S, Swarna SR, Alavandi SV. Isolation of nalidixic acid resistant Campylobacters from cases of paediatric diarrhoea in Chennai. J Commun Dis 1998;30:159-162.
11. Wasfy MO, Oyofo BA, David JC, Ismail TF, el-Gendy AM, Mohran ZS, et al. Isolation and antibiotic susceptibility of Salmonella, Shigella, and Campylobacter from acute enteric infections in Egypt. J Health Popul Nutr 2000;18:33-38.
12. Binotto E, McIver CJ, Hawkins GS. Ciprofloxacin-resistant Campylobacter jejuni infections. Med J Aust 2000;172:244-245.
13. Prasad KN, Mathur SK, Dhole TN, Ayyagari A. Antimicrobial susceptibility and plasmid analysis of Campylobacter jejuni isolated from diarrhoeal patients and healthy chickens in northern India. J Diarrhoeal Dis Res 1994;12:270-273.
14. Hoge CW, Gambel JM, Srijan A Pitarangsi C, Echeverria P. Trends in antibiotic resistance among diarrheal pathogens isolated in Thailand over 15 years. Clin Infect Dis 1998;26:341-345.
15. Huysmans MB, Turnidge JD. Disc susceptibility testing for thermophilic Campylobacters. Pathology 1997;29:209-216.
16. World Health Organization. Use of quinolones in food animals and potential impact on human health. Report and proceedings of a WHO meeting, Geneva, Switzerland, 2-5 June, 1998. Geneva: World Health Organization, 1998.
17. McDermott PF, Bodeis SM, English LL, White DG, Walker RD, Zhao S, et al. Ciprofloxacin resistance in Campylobacter jejuni evolves rapidly in chickens treated with fluoroquinolones. J Infect Dis 2002;185:837-840.
18. Joint Expert Technical Advisory Committee on Antibiotic Resistance (JETACAR). The use of antibiotics in food-producing animals: antibiotic resistant bacteria in animals and humans. Commonwealth Department of Health and Aged Care, Commonwealth Department of Agriculture, Fisheries and Forestry-Australia. Prepared for JETACAR by Biotex Canberra, 1999, Commonwealth of Australia. Available from: http://www.health.gov.au/pubs/jetacar.htm. Accessed: July 2002.
19. Linton D, Lawson AJ, Owen RJ, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrhoeic samples. J Clin Microbiol 1997;35:2568-2572.
20. Harmon KM, Ransom GM, Wesley IV. Differentiation of Campylobacter jejuni and Campylobacter coli by polymerase chain reaction. Mol Cell Probes 1997;11:195-200.
21. Bolton FJ, Wareing DRA, Skirrow MB, Hutchinson DN. Identification and biotyping of Campylobacters. In: Board GR, Jones D, Skinner FA eds. Identification methods in applied and environmental microbiology. Blackwell Scientific Publications, Oxford, United Kingdom, 1992:151-161.
22. National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th edition. Approved standard. National Committee for Clinical Laboratory Standards document M7-A5. Villanova, Pennsylvania, USA, 2001.
23. Reina J, Ros MJ, Serra A. Susceptibilities to 10 antimicrobial agents of 1,220 Campylobacter strains isolated from 1987 to 1993 from feces of pediatric patients. Antimicrob Agents Chemother 1994;38:2917-2920.
24. Wang Y, Huang WM, Taylor DE. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob Agents Chemother 1993;37:457-463.
25. Charvalos E, Tselentis Y, Hamzehpour MM, Kohler T, Pechere JC. Evidence for an efflux pump in multidrug-resistant Campylobacter jejuni. Antimicrob Agents Chemother 1995;39:2019-2022.
26. Ferguson JK, Dalton CB, McGettigan P, Hill S. Antimicrobial resistance in animal enteric bacteria and human disease -a review of the scientific literature. Commissioned report to the Joint Expert Technical Advisory Committee on Antibiotic Resistance (JETACAR). Canberra; National Health and Medical Research Council, 1998.
27. Aquino MH, Filgueiras AL, Ferreira MC, Oliveira SS, Bastos MC, Tibana A. Antimicrobial resistance and plasmid profiles of Campylobacter jejuni and Campylobacter coli from human and animal sources. Lett Appl Microbiol 2002;34:149-153.
Top of page
Author affiliations
1. University of Newcastle and Hunter Area Pathology Service, Newcastle, New South Wales
2. Coordinator, OzFoodNet, Wallsend, New South Wales
3. Elizabeth Macarthur Agricultural Institute, Camden, New South Wales
4. Microbiological Diagnostics Unit, University of Melbourne, Melbourne, Victoria
5. Hunter Public Health Unit, Wallsend, New South Wales
Corresponding author: Dr John Ferguson, HAPS, Locked Bag 1, Newcastle Mail Centre NSW 2310. Telephone: +61 2 4921 4422. Facsimile: +61 2 4921 4440. Email: jferguson@hunter.health.nsw.gov.au
This article was published in Communicable Diseases Intelligence Volume 27 Suppl, May 2003.
